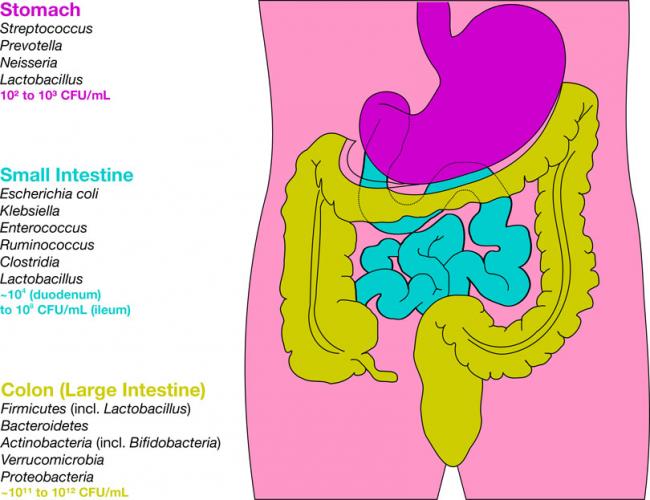
Related Articles
- 08 Jul 20
With pregnancy come major changes in circulation. It’s no wonder: With an increase in body weight from the fetus and surrounding fluid, extra pressure is added onto organs and other structures like the weaker-walled veins. The body’s blood volume also increases and these changes can result in swelling, varicose veins, and hemorrhoids.
- 08 Jul 20
Polycystic ovarian syndrome (PCOS) is one of the most common endocrine disorders. PCOS affects one in every five women of the reproductive age.
- 23 Dec 16
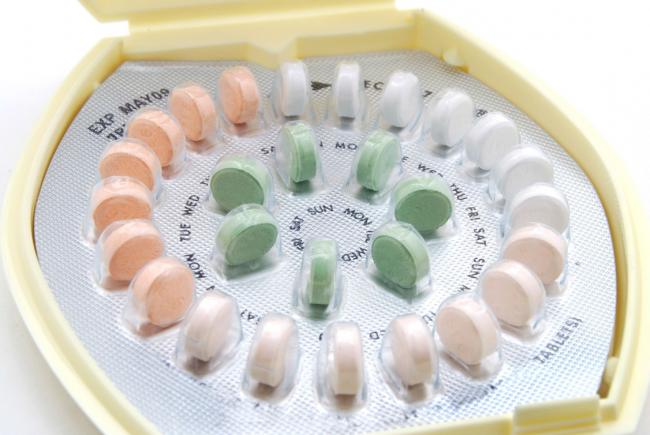
Dies ist Teil 2 unseres zweiteiligen Artikels zu Möglichkeiten der Geburtenkontrolle neben der „Pille“.
Teil 1 behandelte die Hormon- und Kupferspiralen. Teil 2 deckt den Ring, das Pflaster, das Diaphragma, die Kondome und die Rhythmusmethode ab. - 11 Aug 15
 There is a new, growing interest in the field of male infertility, given the recent trends of declining conception over the past decade. The times are telling of families having fewer children, later in age and often having more difficulties with conception. Infertility can pose physical, psychological, financial and economic burden on individuals, the health care system and society.
There is a new, growing interest in the field of male infertility, given the recent trends of declining conception over the past decade. The times are telling of families having fewer children, later in age and often having more difficulties with conception. Infertility can pose physical, psychological, financial and economic burden on individuals, the health care system and society. - 08 Jun 15
 Male fertility is measured by sperm quality and count. A man is considered at risk for infertility if his sperm count is less than 20 million per millilitre, and may be sterile if the sperm count is less than 500,000 per millilitre. Semen analysis is commonly done by light microscope and scanning electron microscope. Female infertility has become a medical hot topic, but what about men; the other half of the equation?
Male fertility is measured by sperm quality and count. A man is considered at risk for infertility if his sperm count is less than 20 million per millilitre, and may be sterile if the sperm count is less than 500,000 per millilitre. Semen analysis is commonly done by light microscope and scanning electron microscope. Female infertility has become a medical hot topic, but what about men; the other half of the equation? - 13 Apr 15
 Pre-eclampsia is a condition that has life-threatening consequences if not treated immediately. If you are a pregnant woman experiencing symptoms of pre-eclampsia, it is important to seek medical attention. Although the cause remains unknown, advancements in research are being made, and several theories exist for the cause of the condition.
Pre-eclampsia is a condition that has life-threatening consequences if not treated immediately. If you are a pregnant woman experiencing symptoms of pre-eclampsia, it is important to seek medical attention. Although the cause remains unknown, advancements in research are being made, and several theories exist for the cause of the condition. - 06 Sep 16
- 17 Aug 16
- 13 Apr 17
- 03 Oct 16
- 06 Oct 14
- 16 Jan 16
- 10 Mar 18
Prenatal genetic testing is used to determine if a fetus has, or is at risk of developing, a genetic disorder. These disorders are caused by changes, often deletions or duplications, in fetal DNA and chromosomes. Two main types of testing often performed are screening and diagnosis. Screening tests typically look for aneuploidy—an abnormal number of chromosomes
- 25 May 16
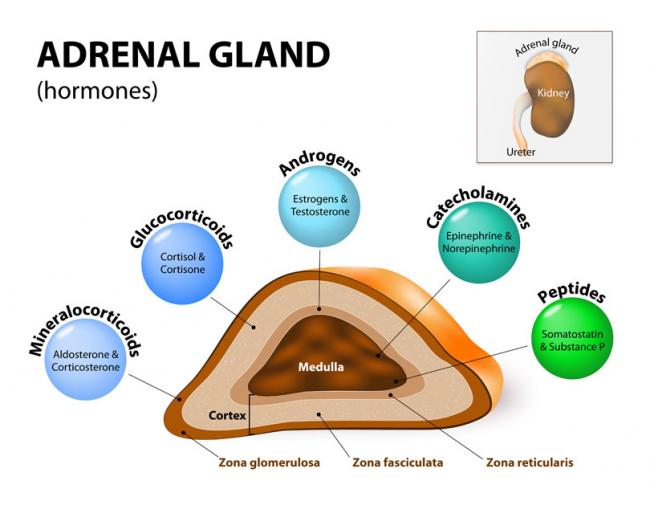
Stressed? In today’s world, we often hear about our adrenal glands being taxed and not performing optimally. Usually, we attribute this to external stressors such as work overload, emotional liabilities, relationship responsibilities, or financial strains. Have you ever thought this could be due to a genetic defect, though?
- 17 May 18
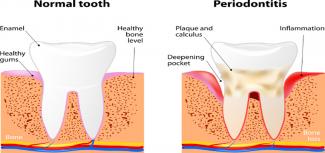
Sure, dental cleaning is great for our oral health, but did you know that it can also improve the wellness of your whole body? Daily toothbrushing and flossing is crucial to maintain the health of our gums and teeth; however, this might actually be more important in pregnant women and those looking to conceive.
- 23 Dec 16
- 25 May 16
 The joy of motherhood can be an exciting and blissful experience. But what if, instead of happiness and joy, the new mom is experiencing weepiness and irritation, and is crying all the time, but does not understand why? What if, instead of excitement at seeing her baby, she has negative feelings and worries that she may hurt her baby?
The joy of motherhood can be an exciting and blissful experience. But what if, instead of happiness and joy, the new mom is experiencing weepiness and irritation, and is crying all the time, but does not understand why? What if, instead of excitement at seeing her baby, she has negative feelings and worries that she may hurt her baby? - 03 Mar 14
 The number of couples experiencing infertility and/or resorting to assisted reproductive technology (ART) is on the rise. A study released in 2012 found that among Canadian couples (women aged 18–44 years), the prevalence of infertility ranged from 11 to 15%, and this was an increase compared to previous statistics. 02 Jun 17
The number of couples experiencing infertility and/or resorting to assisted reproductive technology (ART) is on the rise. A study released in 2012 found that among Canadian couples (women aged 18–44 years), the prevalence of infertility ranged from 11 to 15%, and this was an increase compared to previous statistics. 02 Jun 17 Understanding your menstrual cycle involves more than just estimating your next period. Knowing your body and tracking your menstrual cycles can provide insight into your hormonal and reproductive health. You might be experiencing symptoms that we usually label as “normal,” when we should be calling them “common.”13 Oct 15
Understanding your menstrual cycle involves more than just estimating your next period. Knowing your body and tracking your menstrual cycles can provide insight into your hormonal and reproductive health. You might be experiencing symptoms that we usually label as “normal,” when we should be calling them “common.”13 Oct 15
Newsletter
Most Popular
- 09 Nov 15
- 13 Oct 15
- 17 Jun 13
- 17 Jun 13
- 17 Jun 13
- 01 Jul 13
- 17 Jun 13
- 17 Jun 13
- 17 Jun 13
- 01 Jul 13
- 17 Jun 13
- 17 Jun 13
- 17 Jun 13
- 01 Jul 13