Drug Alert: Risk of blood clots with tofacitinib
Health Canada is conducting a safety review after issues were discovered during a clinical trial involving rheumatoid arthritis patients being treated with the drug tofacitinib (sold in Canada under the brand names Xeljanz and Xeljanz XR).
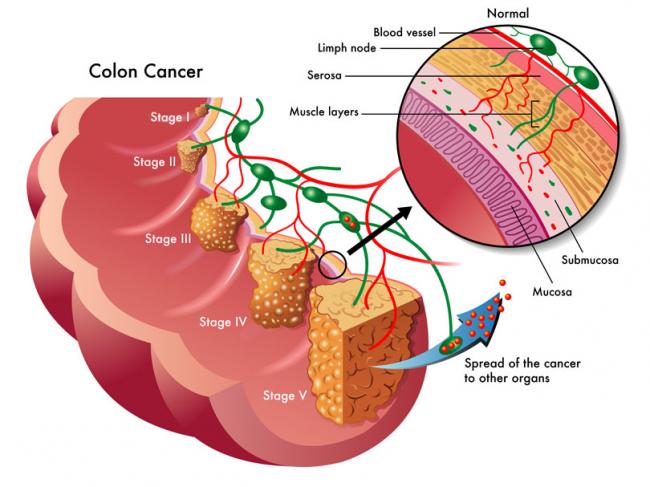
Tofacitinib is a prescription drug used to treat adults with moderate to severely active rheumatoid arthritis, active psoriatic arthritis, or moderate to severely active ulcerative colitis in people who do not respond well to other medications. It is generally prescribed in combination with other drugs, such as methotrexate.
The ongoing clinical trial, run by Pfizer, found an increased risk of blood clots in the lungs and of death when the drug was taken at a high dose of 10 mg, twice a day. Patients who were taking 10 mg of tofacitinib twice a day are now transitioning to the lower, currently authorized dose of 5 mg twice a day.