Related Articles
- 15 Jun 21
Our knowledge of connections between the human microbiome, immune system, and disease continue to evolve. Gut microbiota are influential in the health of our bodies and function to both increase immune-system function and suppress colonization of pathogens.
- 27 Jan 21Emerging health implications of gluten sensitivity reach far beyond the borders of the gastrointestinal tract. The gluten-sensitive spectrum includes different forms of manifestations: allergic (wheat allergy), autoimmune celiac disease (CD), dermatitis herpetiformis, gluten ataxia, and immune-mediated (nonceliac gluten sensitivity).
- 29 Apr 22
Probiotics have extensive potential for therapeutic use, and we continue to discover their specific actions. The previous article looked at classification and the role of probiotics with regards to immune function and digestion, including autoimmunity, atopic skin reactions, and respiratory conditions.
- 12 Feb 20
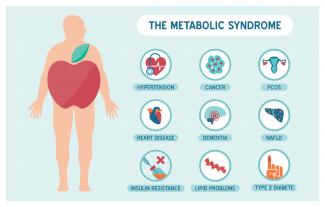
Metabolic syndrome is a cluster of risk factors that increase one’s chances of developing serious illnesses in the future. Metabolic syndrome doubles the chance of developing cardiovascular disease while increasing the risk of diabetes, fatty liver, and several types of cancers.[1][2]
- 10 Jun 20
By now, most of us have heard the word microbiome—the collection of trillions of functional bacteria of all different species found in many places throughout our bodies, such as our skin, gut, mouth, vagina, and lungs.
- 09 Mar 20
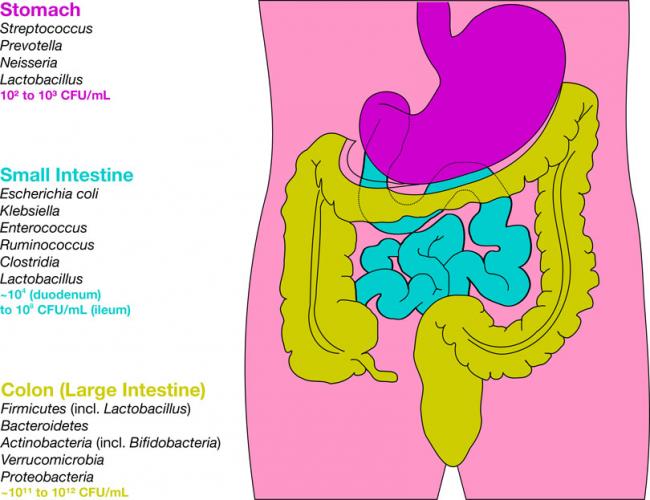
The human gastrointestinal tract (GIT) alone contains 1014 microorganisms including bacteria, viruses, and fungi. That’s approximately 100 times more microbial cells than human cells, which shows how much of an impact they can have on human health.
- 10 Jun 20
In many countries around the world, alcoholic beverages are built into the culture and religion. We consume it as a form of celebration and a form of self-medication. But over the years, it takes a toll on our organ system that works to filter it out. Fatty liver disease is a growing concern in the Western hemisphere, especially as new diagnoses include people of normal body weights and people who don’t drink alcohol.
- 17 Dec 19
Collagen peptides have seemingly appeared out of nowhere. They are being advertised on social media, can be found on the covers of magazines… even my hairdresser has been talking about how she adds collagen peptides to her smoothie! But what are collagen peptides? Why is everyone adding this powder to their coffee, smoothies, or breakfast oatmeal bowl? And the real question is: Should you be adding collagen peptides to your diet as well?
- 02 Jul 14
 Consumption of wheat has increased dramatically over the last decades. Wheat was once thought to be an important part of a healthy diet, but now is seen as a potential health threat for many individuals. Gluten is a food component found in wheat, rye, barley, and cereals. More specifically, it is a protein complex that is formed by gliadin and glutenin, typically used in processed foods as a stabilizing agent. Celiac disease (CD) is a complex chronic immune-mediated disorder whose gastrointestinal symptoms are triggered by eating gluten.
13 Apr 1717 Jun 16
Consumption of wheat has increased dramatically over the last decades. Wheat was once thought to be an important part of a healthy diet, but now is seen as a potential health threat for many individuals. Gluten is a food component found in wheat, rye, barley, and cereals. More specifically, it is a protein complex that is formed by gliadin and glutenin, typically used in processed foods as a stabilizing agent. Celiac disease (CD) is a complex chronic immune-mediated disorder whose gastrointestinal symptoms are triggered by eating gluten.
13 Apr 1717 Jun 16 Crohn’s disease (CD) is a chronic inflammatory disorder of the gastrointestinal tract. It is one of two conditions classified as inflammatory bowel disease (IBD), the other condition being ulcerative colitis (UC). UC is limited to the colon, while CD can involve any segment of the gastrointestinal tract from the mouth to the anus.06 Oct 1616 Jan 16
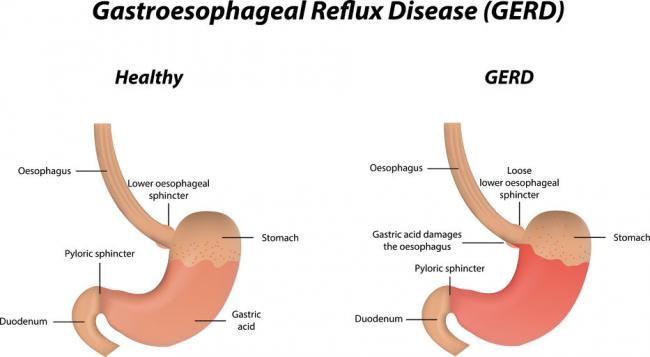
Crohn’s disease (CD) is a chronic inflammatory disorder of the gastrointestinal tract. It is one of two conditions classified as inflammatory bowel disease (IBD), the other condition being ulcerative colitis (UC). UC is limited to the colon, while CD can involve any segment of the gastrointestinal tract from the mouth to the anus.06 Oct 1616 Jan 16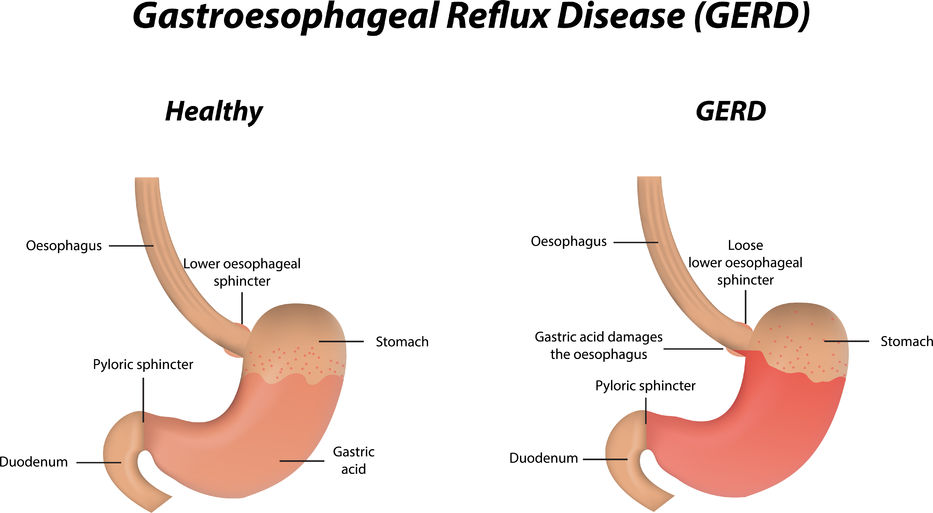 Gastroesophageal Reflux Disease or GERD can be defined as a condition of mucosal damage produced by the abnormal reflux of gastric contents into the esophagus. According to the Canadian Digestive Health Foundation, 5 million Canadians experience heartburn and/or acid regurgitation at least once each week.22 Dec 15
Gastroesophageal Reflux Disease or GERD can be defined as a condition of mucosal damage produced by the abnormal reflux of gastric contents into the esophagus. According to the Canadian Digestive Health Foundation, 5 million Canadians experience heartburn and/or acid regurgitation at least once each week.22 Dec 15 Irritable bowel syndrome (IBS) is a gastrointestinal disorder characterized by irregular bowel habits and abdominal pain. [1] Four subtypes of IBS have been identified: constipation-predominant IBS (IBS-C), diarrhea-predominant IBS (IBS-D), mixed IBS (IBS-M), and un-subtyped IBS. One subtype is not completely independent of the others, for example, patients with IBS-C will most likely experience IBS-D as well at some point in their lives.12 Apr 1713 Feb 16
Irritable bowel syndrome (IBS) is a gastrointestinal disorder characterized by irregular bowel habits and abdominal pain. [1] Four subtypes of IBS have been identified: constipation-predominant IBS (IBS-C), diarrhea-predominant IBS (IBS-D), mixed IBS (IBS-M), and un-subtyped IBS. One subtype is not completely independent of the others, for example, patients with IBS-C will most likely experience IBS-D as well at some point in their lives.12 Apr 1713 Feb 16 The human microbiome is defined as the full collection of genes of all the microbes in the human body and “consists of 10–100 trillion symbiotic microbial cells,” outnumbering the number of human cells by ten times. An individual’s genome is 99.9% identical to another human’s; however, their microbiomes will only show 10–20% similarity to one another.17 Jun 16
The human microbiome is defined as the full collection of genes of all the microbes in the human body and “consists of 10–100 trillion symbiotic microbial cells,” outnumbering the number of human cells by ten times. An individual’s genome is 99.9% identical to another human’s; however, their microbiomes will only show 10–20% similarity to one another.17 Jun 16 The use of complementary medicine for the treatment of cancer and its side effects has skyrocketed in recent years. Complementary therapies refer to ones that are nonpharmaceutical in nature, and that have the potential to not only enhance quality of life, but also to reduce side effects of conventional therapy.07 May 15
The use of complementary medicine for the treatment of cancer and its side effects has skyrocketed in recent years. Complementary therapies refer to ones that are nonpharmaceutical in nature, and that have the potential to not only enhance quality of life, but also to reduce side effects of conventional therapy.07 May 15 The immune system is able to discriminate self from non-self antigens, substances that trigger the immune system, which protects the host from infections and cancer. Autoimmune diseases are characterized by deregulated immune responses. Autoimmune diseases affect 5-8% of the population in the United States and can affect almost every site in the body19 Sep 1711 Oct 18
The immune system is able to discriminate self from non-self antigens, substances that trigger the immune system, which protects the host from infections and cancer. Autoimmune diseases are characterized by deregulated immune responses. Autoimmune diseases affect 5-8% of the population in the United States and can affect almost every site in the body19 Sep 1711 Oct 18A fairly common chronic condition that afflicts people is rheumatoid arthritis (RA); a form of chronic joint inflammation that can have major impact on quality of life. The exact cause of rheumatoid arthritis is unknown, but it has a significant autoimmune component. This is how it differs starkly from the much more common osteoarthritis, which is more of a “wear-and-tear” form of arthritis that results from mechanical use of joints over a long and active life. Rheumatoid arthritis not only affects the joints, but also presents with systemic inflammatory symptoms.
Newsletter
Most Popular
- 09 Jul 15
- 17 Jun 13
- 17 Jun 13
- 17 Jun 13
- 01 Jul 13
- 17 Jun 13
- 17 Jun 13
- 17 Jun 13
- 01 Jul 13
- 17 Jun 13
- 17 Jun 13
- 17 Jun 13
- 01 Jul 13