Environmental Contaminants - Impact on Female Fertility
The number of couples experiencing infertility and/or resorting to assisted reproductive technology (ART) is on the rise.[1] A study released in 2012 found that among Canadian couples (women aged 18–44 years), the prevalence of infertility ranged from 11 to 15%, and this was an increase compared to previous statistics.[2] By comparison, using 1984 data, the prevalence of infertility then was only 5%.[2] Many factors are responsible for this increase, including a delay in childbearing and increased average age of women trying to conceive; compared to 1984, when only 3% of first children were born to mothers aged 35 and older, in 2008, 11% of first-born children were to mothers 35 or older.[2] Other factors known to adversely impact female fertility have also been on the rise, including smoking, alcohol use, and obesity.[2] From 1981 to 2009, the proportion of women aged 20–39 years who are obese has increased from 4 to 21%.[2] Finally, exposure to environmental contaminants may also play a role in this rise in infertility.
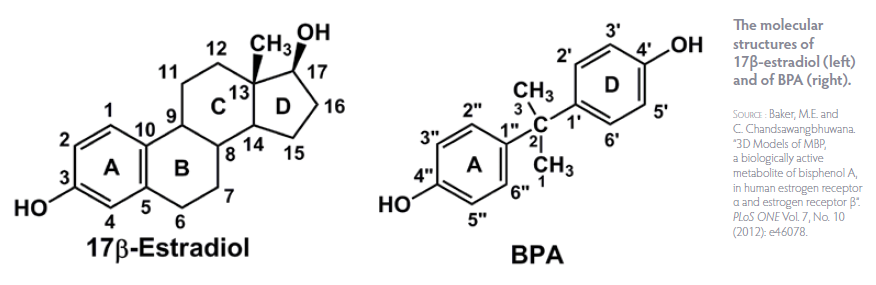
Environmental contaminants may act as endocrine-disrupting chemicals (EDCs), interfering with normal hormone production and signaling. EDCs can mimic or block endogenous hormones, interfering with normal hormone-receptor binding and impairing the expression of target genes, for estrogens and androgen hormones.[3, 4] Studies have shown that exposure to dioxins can result in reduced expression of the progesterone receptor in women with endometriosis,[5] and endometrial tissue resistance to progesterone in animals.[6] A study in young girls found that higher exposure to bisphenol A (BPA) was associated with hypomethylation (resulting in overexpression) of particular genes involved in immune function and inflammation.[7] Thus, in addition to direct effects on hormone systems, EDCs may exert detrimental effects on the immune system, which is also intricately involved in reproductive function.[8] Conditions such as endometriosis, premature ovarian failure, and recurrent miscarriage have all been identified as having autoimmune components.[8, 9]
In 2005, the Collaborative on Health and the Environment assembled researchers from across the United States to participate in a meeting to discuss the impact of environmental contaminants on human fertility.[10] The Vallombrosa Consensus Statement summarized key findings of the meeting (Oct 2005).[11] Importantly, it concluded as likely the fact that “gene-environment interactions are involved in the etiology of many reproductive problems, including impaired sperm quality; PCOS [polycystic ovary syndrome]; endometriosis; uterine fibroids; premature puberty, ovarian failure and menopause; and reproductive cancers”.[11] It also suggested that timing of exposure is important, with potentially the largest impact occurring from exposures before conception, in utero, and in infancy.[11] Shockingly, a recent study identified free BPA present in 62% of breast-milk samples from nursing mothers in the US.[12]
In this article, we will consider the existing evidence on the link between EDC exposure and infertility. In particular, we will consider the impact of bisphenol A (BPA), since this is one of the best-studied EDCs, and to a lesser extent dioxins and polychlorinated biphenyls (PCBs). See Table 1.
Table 1. Endocrine Disruptors (adapted from References 11 & 13)
| Disruptor | Sources |
|---|---|
| Bisphenol A |
Forms the building block of polycarbonate plastics and epoxy resins. Bisphenol A is used in food containers, water bottles, baby bottles, CD cases, eye glass lenses, the lining of food cans, and as a dental sealant. |
| Dioxins | A class of hundreds of related persistent chemicals that result from industrial combustion/incineration processes; burning of household trash or fuels such as wood, coal and oil; chlorine bleaching of pulp/paper; and cigarette smoke. |
| Polychlorinated biphenyls (PCBs) | Persistent, bioaccumulative compounds banned in the US in the late 1970s, but widespread contamination still exists. PCBs were used in hundreds of commercial and industrial applications, including as lubricants, plasticizers, insulators for electrical applications, caulking and paint. |
Endometriosis
Several studies have documented associations between levels of contaminants including dioxins, phthalates, and BPA and risk of endometriosis. Endometriosis is thought to be in large part an estrogen-dominant condition, and it is possible that EDCs such as dioxins, PBCs, and BPA mimic the effects of or increase the sensitivity of endometrial cells to estradiol.[14] In a study of endometrial cells in culture, exposure to BPA resulted in decreased cell proliferation or growth, and increased cell death (apoptosis).[15] BPA may also exacerbate inflammatory mechanisms implicated in endometriosis, including lost sensitivity to progesterone and immune-cell activation.[16]
One study evaluated 17 infertile women and divided them into two groups depending on whether a laparoscopic examination showed evidence of endometriosis or not.[17] Ten women of the 17 had endometriosis. Researchers measured 29 different dioxins in blood and peritoneal (abdominal cavity) fluid. Results showed that higher levels of dioxins (in particular polychlorinated dibenzo-p dioxins, PCDDs, and polychlorinated dibenzofurans, PCDFs) in peritoneal fluid were significantly associated with a 2.5-fold increased risk of endometriosis.[17] Another study found that PCB levels were significantly higher in a group of Italian women with endometriosis, compared with healthy controls.[18] A more recent study by the same research group found a 3- to 6-fold increased odds of having endometriosis among women with elevated blood levels of both non–dioxin-like PCBs as well as dioxin-like PCBs.[19] A recent study of 495 women undergoing laparoscopy and 131 controls found higher levels of phthalates in women with endometriosis. In addition, women with endometriosis not undergoing laparoscopy were shown to have approximately 3-fold higher levels of BPA, 4.19 ng/mL compared to 1.65 ng/mL. Finally, in a study of approximately 70 women, blood testing showed undetectable levels of BPA among healthy women; however, a total of 63% of women with endometriosis showed levels of either bisphenol A or bisphenol B in their blood.[14]
Polycystic Ovary Syndrome (PCOS)
Polycystic ovary syndrome (PCOS) is a disorder affecting up to 10% of women of reproductive age. It is characterized by problems with insulin-glucose regulation called insulin resistance (prediabetes), as well as elevated androgen hormones such as testosterone or androstenedione; both of these factors result in ovulation defects, so that these women may have long, irregular cycles; poor-quality ovulation; and consequent problems with fertility. Research has shown that BPA may be elevated in and may contribute to PCOS.
 In a study of 40 lean and overweight women with PCOS and 20 healthy controls, BPA blood level was independently associated with higher insulin resistance, free androgen index, inflammation, and spleen size (a marker of chronic inflammation).[20] This suggests that not only may BPA worsen hormone imbalances (increasing androgens), but it may also worsen prediabetes (insulin resistance) and inflammation, all of which have a detrimental effect on fertility as well as long-term health. A second study of 71 women with PCOS and 100 healthy controls found that women with PCOS had significantly higher BPA levels: 1.05 vs. 0.72 ng/mL respectively.[21] BPA was also significantly associated with testosterone and androstenedione, two androgen hormones that are elevated in PCOS, and was linked with insulin resistance. Elevated androgens and insulin resistance are two key disturbances that cause ovulation defects in women with PCOS. These studies show that BPA is associated with both of these factors. Intriguingly, there is evidence suggesting a bidirectional relationship between androgens and BPA.[21] Elevated androgens can reduce liver clearance of BPA by inhibiting the enzyme uridine diphosphate-glucuronosyl transferase, and conversely, BPA can displace androgens from their binding protein in the blood (sex hormone-binding globulin, SHBG), thereby increasing the amount of free androgen that is active in the body.[21] It also appears that BPA can directly increase androgen production by the theca cells of the ovary, further exacerbating the problem.[21] A study of 48 Italian women found significantly higher blood levels of BPA in women with infertility, compared to fertile women, in parallel with higher expression of estrogen receptor alpha (ERα) and beta (ERβ), androgen receptor (AR).[22] For instance, BPA was detected in the blood of only three fertile women (23%), compared to 35 (73%, p < 0.01) patients with infertility from any cause (endometriosis or other).[22]
In a study of 40 lean and overweight women with PCOS and 20 healthy controls, BPA blood level was independently associated with higher insulin resistance, free androgen index, inflammation, and spleen size (a marker of chronic inflammation).[20] This suggests that not only may BPA worsen hormone imbalances (increasing androgens), but it may also worsen prediabetes (insulin resistance) and inflammation, all of which have a detrimental effect on fertility as well as long-term health. A second study of 71 women with PCOS and 100 healthy controls found that women with PCOS had significantly higher BPA levels: 1.05 vs. 0.72 ng/mL respectively.[21] BPA was also significantly associated with testosterone and androstenedione, two androgen hormones that are elevated in PCOS, and was linked with insulin resistance. Elevated androgens and insulin resistance are two key disturbances that cause ovulation defects in women with PCOS. These studies show that BPA is associated with both of these factors. Intriguingly, there is evidence suggesting a bidirectional relationship between androgens and BPA.[21] Elevated androgens can reduce liver clearance of BPA by inhibiting the enzyme uridine diphosphate-glucuronosyl transferase, and conversely, BPA can displace androgens from their binding protein in the blood (sex hormone-binding globulin, SHBG), thereby increasing the amount of free androgen that is active in the body.[21] It also appears that BPA can directly increase androgen production by the theca cells of the ovary, further exacerbating the problem.[21] A study of 48 Italian women found significantly higher blood levels of BPA in women with infertility, compared to fertile women, in parallel with higher expression of estrogen receptor alpha (ERα) and beta (ERβ), androgen receptor (AR).[22] For instance, BPA was detected in the blood of only three fertile women (23%), compared to 35 (73%, p < 0.01) patients with infertility from any cause (endometriosis or other).[22]
In Vitro Fertilization
Levels of EDCs, in particular BPA, have been shown to impact ovarian responsiveness in women undergoing in vitro fertilization (IVF). In one study, a total of 44 women undergoing IVF were assessed for their BPA levels.[23] Median unconjugated serum BPA level in the entire cohort was 2.53 ng/mL, and the levels of most women (86%) exceeded the level of detection. Those women with higher levels had lower peak estradiol levels in response to gonadotropin (FSH) stimulation, indicating a poorer ovarian response to hormonal stimulation. In this study, there was no association with antral follicle count, a marker of ovarian reserve; however, in a more recent study, BPA levels were associated with significantly lower antral follicle count, “raising concern for possibly accelerated follicle loss and reproductive aging”.[24]
Similar findings have been reported in a second study assessing urinary BPA.[25] A total of 84 women undergoing a total of 112 IVF cycles were evaluated. The median urinary BPA concentration was 2.61 μg/L, with a range of undetectable up to over 65 μg/L. The study showed that there was an average decrease in estradiol by 213 pg/ml for each log unit increase in BPA. In addition, there was a significant 12% decrease in the number of oocytes retrieved per cycle for each log unit increase in BPA.[25] Peak estradiol was correlated with the number of mature follicles and the number of oocytes retrieved.[23] It appears that BPA may interfere with the synthesis of estrogen by granulosa cells, a supportive cell within the ovary.[23, 26] Data from animal models suggests that BPA inhibits FSH-stimulated granulosa cell aromatase activity, which is required for the conversion of androgens to estrogens within the theca cells of the ovary.[23] BPA has also been shown to cause apoptosis, or programmed cell death, in granulosa cells.[27]
Levels of EDCs have also been linked to IVF outcomes. In one study of 137 women undergoing a total of 180 IVF cycles in a center in the eastern United States, urinary BPA levels were associated with poorer outcomes.[28] Mean urinary BPA levels were 1.53 μg/L. Urine BPA testing is thought to detect exposure over the past weeks to months. A two-fold increased odds of implantation failure were associated with higher urinary BPA concentrations (> 3.80 μg/L), compared with women with the lowest BPA levels (≤ 1.69 μg/L).[28] Implantation is hormonally regulated by estradiol and progesterone, and BPA’s estrogenic effects were thought to interfere with implantation via two possible mechanisms: 1) accelerating the rate of blastocyst development and thus leading to a mismatch in timing with the appropriate uterine receptivity window, and 2) directly decreasing uterine receptivity to blastocyst implantation.[28]
Finally, one study assessed associations between EDCs and changes in DNA methylation. DNA methylation is a mechanism that promotes or silences gene expression; in general, increased methylation decreases expression, while decreased methylation usually leads to increased gene expression. The study found that among women undergoing IVF, those with higher BPA exposure, measured as blood BPA levels, had lower methylation of a gene called TSP50>.[29] The TSP50 gene encodes ‘testes-specific protease 50’, a product of unknown function that is expressed in the testes; nonetheless, this study demonstrates yet another mechanism by which BPA may influence human reproduction, although it is not yet well understood. In this study there was also a correlation between higher mercury levels and increased methylation of the GSTM1/5 promoter, a gene affiliated with glutathione, an important intracellular antioxidant enzyme responsible for detoxifying chemicals and carcinogens.[29]
Conclusion
Collectively, these data demonstrate a potential role for environmental contaminants such as BPA, dioxins, and PCBs in disorders of female reproduction. This association may explain in part the recent rise in fertility-related disorders present in our society. There is a basis for women, infants, and indeed the population in general, to take measures to avoid these common contaminants. A question for future research relates to methods for detection as well as for methods of safe and effective removal of these pollutants from the body, whether through enhancement of glutathione and other detoxification pathways in the body, correction of any nutrient dependencies if applicable, or through strategies that involve sweating such as exercise and infrared sauna therapy.
[collapse collapsed title=References]
1. Sunderam, S., et al.; Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, CDC. “Assisted reproductive technology surveillance — United States, 2010”. Morbidity and Mortality Weekly Report. Surveillance Summaries Vol. 62, No. 9 (2013): 1–24.
2. Bushnik, T., et al. “Estimating the prevalence of infertility in Canada”. Human Reproduction Vol. 27, No. 3 (2012): 738–746.
3. Teng, C., et al. “Bisphenol A affects androgen receptor function via multiple mechanisms”. Chemico-Biological Interactions Vol. 203, No. 3 (2013): 556–564.
4. Li, Y., et al. “Endocrine-disrupting chemicals (EDCs): In vitro mechanism of estrogenic activation and differential effects on ER target genes”. Environmental Health Perspectives Vol. 121, No. 4 (2013): 459–466.
5. Igarashi, T.M., et al. “Reduced expression of progesterone receptor-B in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin”. Fertility and Sterility Vol. 84, No. 1 (2005): 67–74.
6. Bruner-Tran, K.L., T. Ding, and K.G. Osteen. “Dioxin and endometrial progesterone resistance”. Seminars in Reproductive Medicine Vol. 28, No. 1 (2010): 59–68.
7. Kim, J.H., et al. “Bisphenol A–associated epigenomic changes in prepubescent girls: a cross-sectional study in Gharbiah, Egypt”. Environmental Health Perspectives Vol. 12 (2013): 33.
8. Tomassetti, C., et al. “Endometriosis, recurrent miscarriage and implantation failure: is there an immunological link?” Reproductive Biomedicine Online Vol. 13, No. 1 (2006): 58–64.
9. Pires, E.S., et al. “Specific and sensitive immunoassays detect multiple anti-ovarian antibodies in women with infertility”. The Journal of Histochemistry and Cytochemistry Vol. 55, No. 12 (2007): 1181–1190.
10. The Collaborative on Health and the Environment. Vallombrosa Documents. http://www.healthandenvironment.org/infertility/vallombrosa_documents ∙ Accessed 15 Feb 2014.
11. The Collaborative on Health and the Environment. Vallombrosa Consensus Statement on Environmental Contaminants and Human Fertility Compromise — October 2005. http://www.healthandenvironment.org/infertility/vallombrosa_documents ∙ Accessed 15 Feb 2014.
12. Zimmers, S.M., et al. “Determination of free bisphenol A (BPA) concentrations in breast milk of U.S. women using a sensitive LC/MS/MS method”. Chemosphere 2014; pii: S0045-6535(14)00022-8. [Epub ahead of print]
13. Rogers, J.A., L. Metz, and V.W. Yong. “Review: Endocrine disrupting chemicals and immune responses: a focus on bisphenol-A and its potential mechanisms”. Molecular Immunology Vol. 53, No. 4 (2013): 421–430.
14. Cobellis, L., et al. “Measurement of bisphenol A and bisphenol B levels in human blood sera from healthy and endometriotic women”. Biomedical Chromatography Vol. 23, No. 11 (2009): 1186–1190.
15. Bredhult, C., L. Sahlin, and M. Olovsson. “Gene expression analysis of human endometrial endothelial cells exposed to bisphenol A”. Reproductive Toxicology Vol. 28, No. 1 (2009): 18–25.
16. Bruner-Tran, K.L., et al. “Dioxin may promote inflammation-related development of endometriosis”. Fertility and Sterility Vol. 89, No. 5 Suppl. (2008): 1287–1298.
17. Cai, L.Y., et al. “Dioxins in ascites and serum of women with endometriosis: a pilot study”. Human Reproduction Vol. 26, No. 1 (2011): 117–126.
18. Porpora, M.G., et al. “Increased levels of polychlorobiphenyls in Italian women with endometriosis”. Chemosphere Vol. 63, No. 8 (2006): 1361–1367.
19. Porpora, M.G., et al. “Endometriosis and organochlorinated environmental pollutants: a case-control study on Italian women of reproductive age”. Environmental Health Perspectives Vol. 117, No. 7 (2009): 1070–1075.
20. Tarantino, G., et al. “Bisphenol A in polycystic ovary syndrome and its association with liver-spleen axis”. Clinical Endocrinology Vol. 78, No. 3 (2013): 447–453.
21. Kandaraki, E., et al. “Endocrine disruptors and polycystic ovary syndrome (PCOS): elevated serum levels of bisphenol A in women with PCOS”. The Journal of Clinical Endocrinology and Metabolism Vol. 96, No. 3 (2011): E480–E484.
22. Caserta, D., et al. “The influence of endocrine disruptors in a selected population of infertile women”. Gynecological Endocrinology Vol. 29, No. 5 (2013): 444–447.
23. Bloom, M.S., et al. “Bisphenol A exposure reduces the estradiol response to gonadotropin stimulation during in vitro fertilization”. Fertility and Sterility Vol. 96, No. 3 (2011): 672–677.e2.
24. Souter, I., et al. “The association of bisphenol-A urinary concentrations with antral follicle counts and other measures of ovarian reserve in women undergoing infertility treatments”. Reproductive Toxicology Vol. 42 (2013): 224–231.
25. Mok-Lin, E., et al. “Urinary bisphenol A concentrations and ovarian response among women undergoing IVF”. International Journal of Andrology Vol. 33, No. 2 (2010): 385–393.
26. Mlynarcíková, A., et al. “Alterations in steroid hormone production by porcine ovarian granulosa cells caused by bisphenol A and bisphenol A dimethacrylate”. Molecular and Cellular Endocrinology Vol. 244, No. 1–2 (2005): 57–62.
27. Xu, J., et al. “Bisphenol A induces apoptosis and G2-to-M arrest of ovarian granulosa cells”. Biochemical and Biophysical Research Communications Vol. 292, No. 2 (2002): 456–462.
28. Ehrlich, S., et al. “Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization”. Environmental Health Perspectives Vol. 120, No. 7 (2012): 978–983.
29. Hanna, C.W., et al. “DNA methylation changes in whole blood is associated with exposure to the environmental contaminants, mercury, lead, cadmium and bisphenol A, in women undergoing ovarian stimulation for IVF”. Human Reproduction Vol. 27, No. 5 (2012): 1401–1410.
[/collapse]